(a) State the following laws of chemical combination: (i) Law of constant composition (ii) Law of multiple’ proportion.
(b) Copper reacts with oxygen to form two oxides X and Y. On analysis, 1.535 g of X yielded 1.365 g copper and 1.450 g of Y yielded 1.160 g of cooper.
i) Determine the chemical formula of X and Y.
(ii) Calculate the mass of copper which can react with 0.500 g of oxygen to yield I. X II. Y.
(iii) Which of the laws of chemical combination is illustrated by the result in (b)(i) above. [ = 16, Cu = 63.51]
(c) Write the structure of the product responsible for the observation in each of the following reactions:
(i) A mixture of butanoic acid and ethanol warmed in the presence of concentrated H\(_2\)SO\(_4\) gives off a fragrant odour.
(ii) Sodium dissolves in propan-2-ol with effervescence to give a solution which on evaporation to dryness leaves a white precipitate.
(d) Consider the compound CH\(_3\)CH\(_2\)COOCH\(_2\)CH\(_3\).
(i) Name the compound (ii) Write the structural formula of the compound (iii) State the reagents and conditions for the formation of the compound.
(a)(i) State two differences betwecii the properties of solids and gases
(ii) What process does each of X, Y and Z represent in the changes shown below?
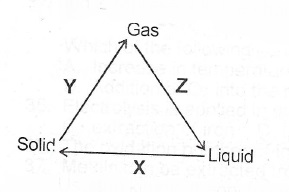
(b)(i) State Charles’ Law (ii) Draw a sketch to graphically illustrate Charles’ Law.
(c) 60 cm of hydrogen diffused through a porous membrane in 10 minutes. The same volume of a gas G diffused through the same membrane in 37.4 minutes. Determine the relative molecular mass of G. [ H = I ]
(d)(i) State two assumptions X of the kinetic theory.
(ii) Consider the reaction represented by the Solid of Liquid following equation:
H\(_{2(g)}\) + Cl\(_{2(g)}\) \(\to\) 2HCI\(_{(g)}\)
Use the kinetic theory to explain how the rate of formation of HCI\(_{(g)}\) would be affected by I. increase in temperature; II. decrease in pressure.
(e) Given different examples, mention one metal in each case vihich produces hydrogen on reacting with (i) dilute mineral acid; (ii) cold water; (iii) steam; (iv) hot, concentrated alkali.
a) Define each of the following terms and indicate one use of each:
(i) Nuclear fission; (ii) Nuclear fusion.
(b) Alpha particle emission by \(^{293}_{25}U\) proceduces an element A. Beta particle emission by the particle A produces another element B. Element B also undergoes alpha particle emission to produce \(^{227}_{89}AC\). Write balanced equations to represent the above statement.
(c) The models below represent the filling of orbitals in an atom.
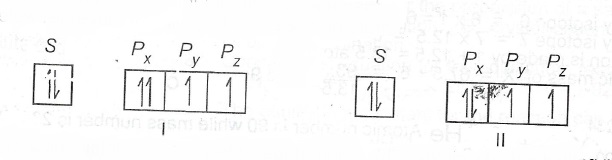
State which rule(s) is/are violated or obeyed by each model.
(d) Explain why the boiling point of H\(_2\)S with relative molecular mass of 34 is lower than that of H\(_2\)O with relative molecular mass of 18.
(e) HCI is passed into each of the following solvents:
(i) water;
(ii) methylbenzene. I. State the effect of each solution on blue litmus paper II. Compare the electrical conductivities of the two solutions.
(f) Zinc dust is added to copper (II) tetraoxosulphate (VI) solution. State;
(i) what is observed; (ii) the type of reaction that occurs.
(a)(i) Draw and label a diagram to illustrate the preparation and collection of dry chlorine gas in the laboratory.
(ii) List two uses of chlorine.
(b)(i) Explain why river water flowing through an industrial town may be unsafe for drinking.
(ii) State the use of each of the following substances in water treatment: I. Sand, II. Chlorine, III. Calcium oxide, IV. Alum
(c)Consider the reaction represented by the following equation:
2Na\(_2\)CI\(_{(s)}\) H2SO4(aq) \(\to\) Na\(_2\)SO\(_{(4(aq)}\) + 2HCI\(_{(g)}\)
Calculate the volume of HCI gas that can be obtained at s.t.p. from 5.85 g of sodium chloride. [H = 1, Na = 23, CI = 35.5, Molar volume a 22.4 dm\(^3\) at s.t.p]
(d) Give one example in each case of a (i) metal that is a liquid at room temperature. (ii) non-metal that is a iiquid at room temperature, (iii) gas at room temperature that is monatomic.
(e) State two differences between metals and nom metals with respect to their: (i) physical properties; (ii) chemical properties.
(a) A solution of CuSO\(_4\) was electrolyzed between pure copper electrodes and the following results were obtained:
Mass of copper anode before experiment = 7.20 g
Mass of copper anode after experiment = 4.00 g
Mass of copper cathode before experiment = 5.75 g
From the information provided,
(i) calculate the mass of the cathode, after the experiment.
(ii) write an equation for the reaction at the I. anode, II. cathode.
(iii) state whether the colour of the solution would change during the electrolysis. Give a reason for your answer.
(iv) if the electrolysis was carried out for 1 hour 20 minutes with a current of 2.0 amperes, determine the value of the Faraday.
(b) Consider the reaction represented by the following equation:
MnO\(^-_4\) + I\(^-\) + H\(^+\) \(\to\) I\(_2\) + H\(_2\)O + Mn\(^{2+}\)
Write balanced half equation for the (i) oxidation reaction, (ii) reduction reaction.
(c)(i) Describe briefly how tin can be extracted from its ore.
(ii) State one use of tin.
(iii) Mention one property that makes tin suitable for the use stated in (c)(ii)
(d)(i) What is meant by the term pollution?
(ii) Explain why it is dangerous to run a generator in a closed room.
(a) State the following laws of chemical combination: (i) Law of constant composition (ii) Law of multiple’ proportion.
(b) Copper reacts with oxygen to form two oxides X and Y. On analysis, 1.535 g of X yielded 1.365 g copper and 1.450 g of Y yielded 1.160 g of cooper.
i) Determine the chemical formula of X and Y.
(ii) Calculate the mass of copper which can react with 0.500 g of oxygen to yield I. X II. Y.
(iii) Which of the laws of chemical combination is illustrated by the result in (b)(i) above. [ = 16, Cu = 63.51
(c) Write the structure of the product responsible for the observation in each of the following reactions:
(i) A mixture of butanoic acid and ethanol warmed in the presence of concentrated H\(_2\)SO\(_4\) gives off a fragrant odour.
(ii) Sodium dissolves in propan-2-ol with effervescence to give a solution which on evaporation to dryness leaves a white precipitate.
(d) Consider the compound CH\(_3\)CH\(_2\)COOCH\(_2\)CH\(_3\).
(i) Name the compound (ii) Write the structural formula of the compound (iii) State the reagents and conditions for the formation of the compound.
(a)(i) State two differences betwecii the properties of solids and gases
(ii) What process does each of X, Y and Z represent in the changes shown below?
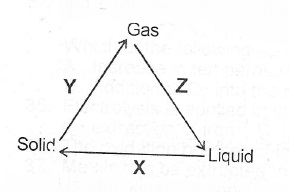
(b)(i) State Charles’ Law (ii) Draw a sketch to graphically illustrate Charles’ Law.
(c) 60 cm of hydrogen diffused through a porous membrane in 10 minutes. The same volume of a gas G diffused through the same membrane in 37.4 minutes. Determine the relative molecular mass of G. [ H = I ]
(d)(i) State two assumptions X of the kinetic theory.
(ii) Consider the reaction represented by the Solid of Liquid following equation:
H\(_{2(g)}\) + Cl\(_{2(g)}\) \(\to\) 2HCI\(_{(g)}\)
Use the kinetic theory to explain how the rate of formation of HCI\(_{(g)}\) would be affected by I. increase in temperature; II. decrease in pressure.
(e) Given different examples, mention one metal in each case vihich produces hydrogen on reacting with (i) dilute mineral acid; (ii) cold water; (iii) steam; (iv) hot, concentrated alkali.
a) Define each of the following terms and indicate one use of each:
(i) Nuclear fission; (ii) Nuclear fusion.
(b) Alpha particle emission by \^293_25U\) proceduces an element A. Beta particle emission by the particle A produces another element B. Element B also undergoes alpha particle emission to produce \(^227_89AC\). Write balanced equations to represent the above statement.
(c) The models below represent the filling of orbitals in an atom.
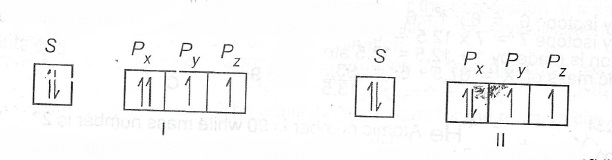
State which rule(s) is/are violated or obeyed by each model.
(d) Explain why the boiling point of H\(_2\)S with relative molecular mass of 34 is lower than that of H\(_2\)O with relative molecular mass of 18.
(e) HCI is passed into each of the following solvents:
(i) water;
(ii) methylbenzene. I. State the effect of each solution on blue litmus paper II. Compare the electrical conductivities of the two solutions.
(f) Zinc dust is added to copper (II) tetraoxosulphate (VI) solution. State;
(i) what is observed; (ii) the type of reaction that occurs.
(i) Draw and label an energy profile diagram of an endothermic reaction. Indicate on your diagram the I. activation energy II. heat change, \(\Delta\)H.
(ii) Explain how the rate of reaction is affected by I. addition of a catalyst, II. increase in temperature.
(b)(i) Write an equation for the thermal decomposition of calcium trioxocarbonate (IV)
(ii) Determine the volume of carbon (IV) oxide measured at s.t.p. that would he produced by the thermal decomposition of 10g calcium trioxocarbonate (IV). [ Ca = 40; O = 16; C = 12 ]
(c)(i) Give one use cf each of the following forms of carbon: I. coal; II. wood charcoal; Ill. carbon (IV) oxide.
(ii) Write a balanced chemical equation to show what happens when each of the following compounds is heated strongly: I. NaNO\(_{3(s)}\) II. MgCO\(_{3(s)}\).
(d) Consider the following compounds: CaO, CaCO\(_3\), Ca(OH)\(_2\), NaOH Which of them is
(i) used in the manufacture of cement;
(ii) used to detect the presence of carbon (IV) oxide;
(iii) used to liberate carbon (IV) oxide when dilute acid is added;
(iv) hygroscopic;
(v) deliquescent?
i) What is the name of the process used for the industrial preparation of tetraoxosulphate (VI) acid?
(ii) State the catalyst used in (a)(i)
(iii) Show by means of bdanced chemical equations only, the industrial preparation of tetraoxosulphate (VI) acid from sulphur (IV) oxide.
(b)(i) Distinguish between dehydration and drying
(ii) Explain why concentrated tetraoxosulphate (VI) acid cannot be used to dry ammonia
(iii) What is the drying agent for ammonia?
(c)(i) Give one example of I. a chloride which is soluble in hot water, II. a trioxocarbonate (IV) which does not decompose on heating, Ill. an amphoteric oxide
(ii) List three methods for the preparation of salts
(iii) State one method for the recovery of salt from its solution.
(d)(i) State Gay Lussac’s law of combining volumes
(ii) Consider the reaction represented by the following equation: C\(_2\)H\(_{4(g)}\) + 3O\(_{2(g)}\) \(\to\) 2H\(_2\)O\(_{(g)}\) + 2CO\(_{2(g)}\). What is the volume of oxygen required for the complete combustion of 12.5 cm\(^{3}\) of ethene?
(a) Write an equation in each case to represent the (i) \(\beta\)-decay of \(^{24}_{11}Na\) to give Mg.
ii) reaction of sodium with cold water.
b)(i) State two differences between reaction (a)(i) and (ii)
ii) State two applications of the type of reaction represented in(a)(i)
(c) Consider the reaction represented by the equation:
Mg\(_{(s)}\) + 2HCI\(_{(aq)}\) \(\to\) MgCl\(_{(aq)}\) + H\(_{(q)}\)
(i) Name the type of reaction involved
(ii) Give two ways by which the reaction could be made faster.
(iii) What volume of hydrogen gas would be produced from 6.0 g of the magnesium? [ H = 1; 1 mole of gas occupy 22.4 dm\(^3\) at s.t.p. ]
(d) What is (i) an electrolyte?; (ii) electrolysis?
(e)(i) Give one metal that is extracted using electrolytic process.
(ii) Name the ore of the metal.
(iii) What is the substance discharged at each electrode when dilute NaCI is electrolysed using graphite electrodes?
(iv) Why would aqueous NaCI conduct electricity but solid NaCI would not?
(v) Give one industrial use of NaCI.
(a)(i) What is 2 homologous series?
(ii) Give two homologous series present in petroleum
(iii) Give one example of a compound belonging to each of the homologous series in (a)(ii).
(iv) Name two fractions obtained from the fractional distillation of petroleum
(v) Why is there a gradual change in the physical properties of petroleum fractions?
b) Write a two-step balanced chemical equation for the reaction of (i) ethanol with excess concentration traoxosulphate (VI) acid at high temperature
(ii) excess ethanol with concentrated tetraoxosulphate (VI) acid at lower temperature.
(c) An organic compound of relative molecular mass 46, on analysis vas ound to contain 52.0% carbon, 13.3% hydrogen and 34.7% oxygen.
(i) Determine its I. empirical formula, II. molecular formula.
(ii) Draw two possible structures of the compound and name one of them [O = 16; C = 12; H = 1 ]
a) Define the following in term:. of electron transfer: (i) oxidation; (ii) reduction.
(b)(i) Determina the oxidation stale of phosphorus in each of the following structures: I. POCI\(_3\) II. PH\(_3\).
(ii) State with reasons whether the following compounds will form acidic, neutral or basic aqueous solutions: I. NaNO\(_3\) II. Na\(_2\)H\(_4\)CI; Ill. Na\(_2\)CO\(_3\).
(c) Consider the set-up
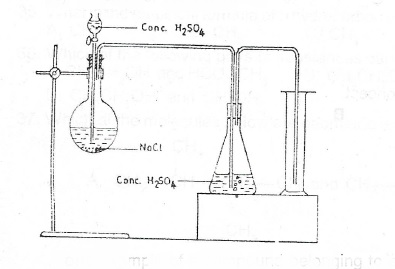
(i) What is the gas produced in the experiment illustrated by the set-up above?
(ii) Name the method of collection of gas
(iii) Give a reason for your answer in (c)(ii) above
(iv) State the function of the concentrated H\(_2\)SO\(_4\) in the conical flask
(v) Give of collection of the gas one I. physical property; II. chemical property of the gas
(vi) State one chemical test to identify the gas.
(d) A 4.3 g hydrated sodium tetraoxosulphate (VI) (Na\(_2\)SO\(_4\).xH\(_2\)O) was heated to remove the water of crystallization. The remaining anhydrous salt had a mass of 2.12 g. Calculate the value of x in the t I hydrated salt. [H = 1; O = 16; Na = 23; S = 32 ]
(a) Define the following terms:(i) Saturated solution; (ii) Solubility.
(b) In an experiment to determine the solubility of a given salt Y, the following data were provided:
Mass of dry empty dish = 7.16 g
Mass of dish + saturated solution of salt Y = 17.85 g
Mass of dish + salt Y = 9.30 g Temperature of solution = °C
Molar mass of salt Y = 100
Density of solution Y = 1.00 g cm\(^{-3}\) Calculate the solubility of salt Y in
(i) g dm\(^{-3}\) of solution, (ii) mol dm\(^{-3}\) of solution.
(c) State the type of bond broken on melting each of the following substances: (i) NaCI\(_{(s)}\) (ii) CO\(_{2(s)}\) (iii) SiO\(_{2(s)}\) (iv) Al\(_{(s)}\)
(d) Explain the following observations: (i) the chemical reactivity of alkali metals increases down the group; (ii) Mg has higher melting point than Na; (iii) K is a better reducing agent than Na.
(e)(i) What are isotopes? (ii) Lithium exists as \(^6_3\)Li and \(^7_3\)Li in the ratio 2:25. Calculate the relative atomic mass of the lithium.
(a)(i) Define hard water
(ii) Name two substances responsible for hardness in water
(iii) Give two methods for the removal of hardness in water.
(b)(i) What are the raw materials require for the manufacture of tetraoxosulphate (VI) acid by the contact process?
(ii) Write an equation for tr reaction that requires a catalyst in the contact process (iii) State the catalyst used in (b)(ii).
(c)(i) Give two uses of sodium tetraoxosulphate (VI) Consider the reaction represented by the following equation:
Na\(_2\)SO\(_4\).10H\(_2\)O\(_{(s)}\) \(\to\) Na\(_2\)SO\(_4\).H\(_2\)O + 9H\(_2\)O\(_{(g)}\)
(ii) What name is given to this type of reaction?
(iii) Calculate the solubility of Na\(_2\)CO\(_3\) at 25°C. if 30.0 cm\(^3\) of its saturated solution at that temperature gave 1.80 g of the anhydrous salt. [C = 12, 0 = 16, Na = 23]
(d)(i) Define the term activated complex
(ii) State one reason why a collision may not produce a chemical reaction
(iii) The formation of water gas is represented by the following equation:
C\(_{(s)}\) + H\(_2\)O\(_{(g)}\) \(\to\) CO\(_{(g)}\) + H\(_{2(0)}\) \(\Delta\)H= + 131 kJmol\(^{-1}\)
Draw an energy profile diagram for the reaction showing the I. activated complex, II. enthalpy of reactants.
(a)(i) What are acidic oxides?
(ii) Give one example of each of the following oxides: I. acidic oxide; II. basic oxide; III. amphoteric oxide; IV. neutral oxide
(b)(i) Define each of the following terms; I. Heat II. Heat of neutralization
(ii) Weite the above an equation to illustrate each of the terms in (b)(i) above
(ii) Given that the standard heat of combustion of butane (C\(_4\)H\(_{(10)}\) is + 5877 kJmol\(^{-1}\), calculate the heat of 14.5 g butane. [ H = 1, = 12 ]
(c) (i) Name two allotropes of sulphur
(ii) State one difference between the two allotropes.
(d)(i) Give two characteristics of noble eases
(ii) State one use each of I. He; II. Ar.
(e) State what is observed on warming ammonium trioxonitrate (V) with sodium hydroxide.
(a)(1) Define covalent bond.
(ii) Give two properties of covalent compounds
(ii) With the aid of a diagram, show how ammonia molecule is formed
(iv) Illustrate with a diagram the formation of ammonium ion?
(v) What type of bond(s) exist(s) in I. ammonia, H. ammonium ion? (\(_1\)H\(_7\)N)
(b)(i) Write three subatomic particles with their corresponding relative masses. CH\(_2\)OH
(ii) Name the possible states in which water can exist.
(c) (i) State Graham’s law of diffusion
(ii) Arrange the following gases, He, CH\(_4\) and N\(_2\) in order of increasing rates of diffusion. Give a reason for the order. [ H = 1, He = 4, C = 12, N = 14 ]
(d) Draw the structures of the following compounds:
(i) 2,3-dimethylbutane;
(ii) 1,4-dibromocyclohexane.
(a) Consider the following reaction sequence.
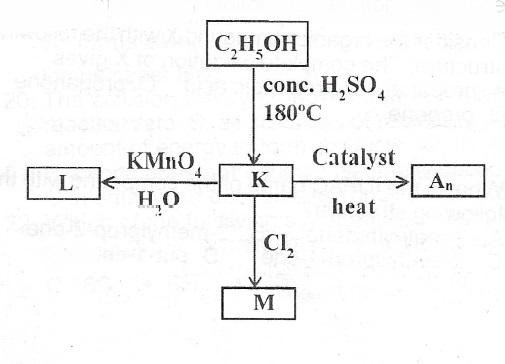
(i) What process leads/tathe formation of K?
(ii) Write the formula of K.
(iii) Write the structural formula of L and name L.
(iv) Name A\(_n\)
(v) Write the structure of M and name M.
(b)(i) What are carbohydrates?
(ii) Give one example each of a I. monosaccharide; II. disaccharide; III. polysaccharide.
(c) Consider the following structure of a simple sugar :
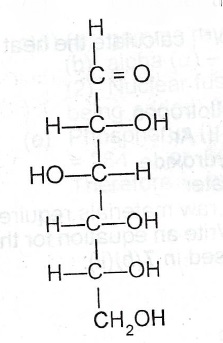
(i) Which functional group makes the compound a reducing agent?
(ii) State what would C = O be observed when I. the compound is mixed with Fehling’s solution and boiled;
I. few drops of concentrated H\(_2\)SO\(_4\) is added to the sample of the compound. H—C—OH
(iii) Write an equation for the reaction in (c)(ii)(II).
(d) A hydrocarbon Z with molecular mass 78 on combustion gave 3.385 g of CO\(_2\) and and 0.692 g of H\(_2\)O. Determine the molecular formula of Z. [ H = 1, C = 12, O = 16]
(a)(i) Define in terms of electron transfer I. oxidizing agent; II. reducing agent.
(ii) Write a balanced equation to show that carbon in a reducing agent.
(iii) State the change in oxidation number of the specie that reacted with carbon in (a)((ii).
(b) A gas X has a vapour density of 32. It reacts with sodium hydroxide solution to form salt and water only. It decolourizes acidified potassium tetraoxomanganate (VII) solution and reacts with H\(_2\)S to form sulphur. Using the information provided:
(i) identify gas X; (ii) state two properties exhibited by X;
(iii) give two uses of X.
(c) Consider the following substances: sodium; lead (II) iodide; hydrogen; magnesium; oxygen. Which of the substances
(i) conducts electricity?
(ii) is produced at the cathode during electrolysis of H\(_2\)SO\(_{4(aq)}\)?
(iii) corresponds to the molecular formula A\(_2\)?
(iv) is an alkaline earth metal?
d)(i) Define the term salt.
(ii) Mention two types of salt
(iii) Give an example of each of the salts mentioned in (d)(ii) above.
(e) In a neutralization reaction, dilute tetraoxosulphate (VI) acid completely reacted with sodium hydroxide solution.
(i) Write a balanced equation for the reaction
(ii) How many moles of sodium hydroxide would be required for the complete neutralization of 0.50 moles of tetraoxosulphate (VI) acid?
(a) State (i) Pauli’s Excusion principle;
(ii) Hund’s rule of maximum multiplicity.
(b)(i) Write the electronic configuration of each of the following ions of copper: I. Cu\(_+\) II. Cu\(_{(2+)}\) [\(_{29}Cu\)]
(ii) Give the number of unpaired-electrons in each of the ions in (b)(i) above.
(iii) State the type of reaction represented by the following equation:
2Cu\(^+_{(aq)}\) \(\to\) Cu\(^{2+}_{(aq)}\) + Cu\(_{(s)}\)
(iv) Write the formula of one compound of Cu+.
(c)(i) Name the type of radiation that will I. penetrate lead block; II. be stopped by thin paper
(ii) Give the charge on each of the radiations mentioned in I(c)(i) above.
(iii) What term is used to describe each of the following nuclear processes?
I. Combination of two lighter nuclei to form a heavy nucleus II. Splitting of a heavy nucleus into two or more lighter nuclei
III. Time required for one-half of the atoms of a radioactive substance to decay.
(d) Arrange the following ions in order of increasing size. Give a reason for your answer in each case. I. Li\(^{+}\), K\(^{+}\), Na\(^{+}\); II. O\(^{2-}\), F\(^{-}\), N\(^{3-}\)
(e) Determine the percentage composition of phosphorus and oxygen in phosphorus (V) oxide [ P = 31, O = 16 ]
(a) State Gay Lussac’s Law.
(b) Carbon (II) oxide reacted with oxygen to form carbon (IV) oxide in a see tube.
(i) Write a balanced equation for the reaction.
(ii) If 40 cm\(^3\) of the carbon (II) oxide were mixed with cm\(^3\) of oxygen,
I. calculate the volume of carbon (IV) oxide produced
II. which reactant is in excess and how much?
III. what was the total volume of the gaseous mixture at the end of the reaction?
(c) Consider the following oxides: CaO, SiO\(_2\), CO, NO\(_2\) and ZnO. Which of the oxide(s)
(i) is an acidic oxide that is insoluble in water?
(ii) reacts with water to give alkaline solution?
(iii) is amphoteric?
(iv) is neutral?
(v) is/are gaseous at room temperature?
(d) Explain why
(i) colourless concentrated trioxonitrate (V) acid turns yellow,
(ii) dilute trioxonitrate (V) acid does not liberate hydrogen when it reacts with magnesium.
(e) Write a chemical equation for the thermal decomposition of (i) Cu(NO\(_3\))\(_{2(g)}\)
(ii) NH\(_4\)NO\(_{3(g)}\)

