The principle which states that no two electrons in the same orbitals of an atom have same value for all four quantum numbers is the
- A. Aufbau principle
- B. Hund's rule
- C. Pauli Exclusion principle
- D. Dilution principle
What method is suitable for the separation of gases present in air?
- A. Catalytic cracking of liquid air
- B. Fractional distillation of liquid air
- C. Thermal decomposition of air
- D. Catalytic decomposition of air
CuO\(_s\) + H\(_2\)(\(_g\)) ⇌ Cu\(_s\) + H\(_2\)O(\(_g\))
In the equation above, the effect of increased pressure on the equilibrium position is that
- A. the equilibrium is shifted to the left
- B. the equilibrium is shifted to the right
- C. there is no effect
- D. more H\(_2\)\((g\)) is produced
One of the following is not a water pollutant?
- A. Inorganic fertilizers
- B. Warm water affluent
- C. Oxygen gas
- D. Biodegradable waste
The ions responsible for permanent hardness in water are sulphates of
- A. Fe\(^{3+}\) and Mg\(^{2+}\)
- B. Ca\(^{2+}\) and Mg\(^{2+}\)
- C. Fe\(^{2+}\) and Fe\(^{3+}\)
- D. Na\(^{+}\) and Ca\(^{2+}\)
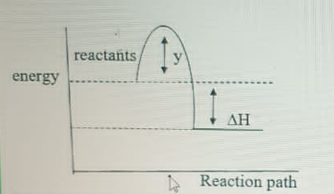
In the graph above, y represents
- A. endothermic reaction
- B. activation energy
- C. exothermic reaction
- D. ionization energy
Which of these is the most preferred separation technique for the isolation of solutes where the purity of the constituent is of utmost importance?
- A. sieving
- B. Distillation
- C. recrystallization
- D. Precipitation
How many moles of CO\(_2\) are produced when ethanol is burnt with 6g of oxygen
- A. 0.125
- B. 0.250
- C. 0.375
- D. 0.750
For chemical reaction to be spontaneous, ∆G must be
- A. positive
- B. negative
- C. zero
- D. equal to the enthalpy change
The chemical formula for potassiumhexacyanoferrate(II) is
- A. [Fe(CN)\(_6\)]\(^{4-}\)
- B. K\(_3\)Fe(CN)\(_6\)
- C. Fe(CN)\(_6\)
- D. K\(_4\)Fe(CN)\(_6\)
Strong acids can be distinguished from weak acids by any of the following methods, EXCEPT
- A. Conductivity measurement
- B. The use of litmus paper
- C. Measurement of pH
- D. Measurement of heat of reaction
The pH of a 0.001 mol dm\(^{-3}\) of H\(_2\)SO\(_4\) is
[Log\(_{10}\)2 = 0.3]
- A. 2.7
- B. 3.0
- C. 3.3
- D. 2.0

The table above shows the formulae of some ions. In which of these compounds is the formula not correct?
- A. Aluminiumtetraoxosulphate(VI), Al\(_2\)(SO\(_4\))\(_3\)
- B. Calciumtrioxonitrate(V), Ca(NO\(_3\))\(_2\)
- C. Iron(III)bromide, Fe\(_3\)Br
- D. Potassiumsulphide, K\(_2\)S
What would be the order of the electrolytic cell in an industry intending the production of silver plated spoons?
- A. Cathode is the spoon; anode is a silver rod; electrolyte is a soluble silver salt
- B. Cathode is a silver rod; anode is the spoon; electrolyte is a soluble silver salt
- C. Cathode is the spoon; anode is any rod; electrolyte is a soluble silver salt
- D. Cathode is any rod; anode is the spoon; electrolyte is a soluble salt
The quantity of electricity required to deposit 180g of Ag from a molten silver trioxonitrate(V) is
[Ag = 108]
- A. 1.08F
- B. 3.30F
- C. 1.67F
- D. 1.80F
127g of sodium chloride was dissolved in 1.0dm\(^3\) of distilled water at 25\(^0\)C . Determine the solubility in moldm\(^{-3}\) of sodium chloride at that temperature. [Na = 23, Cl = 35.5]
- A. 1.0
- B. 2.0
- C. 2.2
- D. 4.1
If the solubility of KNO\(_3\) at 30\(^0\)C is 3.10 mol/dm\(^3\) a solution containing 303g/dm\(^3\) KNO\(_3\) is likely to be
- A. saturated
- B. unsaturated
- C. supersaturated
- D. at saturation point
When a few drops of Millon reagents is added to egg-white solution in a test tube, the white precipitate changes to
- A. orange
- B. brick red
- C. reddish brown
- D. blue
How many isomers has the organic compound represented by the formula C\(_3\)H\(_8\)O ?
- A. 2
- B. 3
- C. 4
- D. 5
A gas when mixed with oxygen, it produces a very hot and early controllable flame. What is the name of the flame and where is it used?
- A. Acetylene flame; miners' lamb
- B. Oxy-ethylene ; hunters' torch
- C. Oxy-ethylene flame; miners' lamb
- D. Oxy-ethylene flame ; cutting and welding metals


