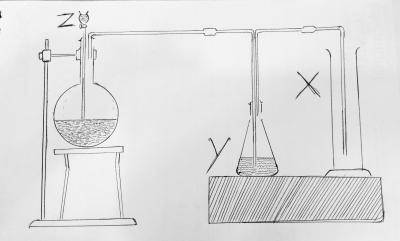
An oxide XO2 has a vapour density of 32. What is the atomic mass of X?
- A. 20
- B. 32
- C. 14
- D. 12
According to Charle’s law, the volume of a gas becomes zero at
- A. −100°c
- B. −273°c
- C. −373°c
- D. 0°c
The carbon atoms on ethane are
- A. sp2 hybridized
- B. sp3 hybridized
- C. sp4 hybridized
- D. sp hybridized
The densities of two gases, X and Y are 0.5gdm-3 and 2.0gdm-3 respectively. What is the rate of diffusion of X relative to Y?
- A. 0.1
- B. 0.5
- C. 2.0
- D. 4.0
The reddish–brown rust on iron roofing sheets consists of
- A. Fe3 + (H2O)6
- B. FeO.H2O
- C. Fe2O3.XH2O
- D. Fe3O4.2H22O
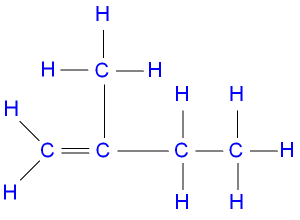
The IUPAC nomenclature of the structure above is
- A. 3-methybut-3-ene
- B. 2-methylbut-1-ene
- C. 2-ethylprop-1-ene
- D. 2-methylbut-2-ene
Calculate the mass of copper deposited when a current of 0.5 ampere was passed through a solution of copper(II) chloride for 45 minutes in an electrolytic cell. [Cu = 64, F = 96500Cmol-1]
- A. 0.300g
- B. 0.250g
- C. 0.2242g
- D. 0.448g
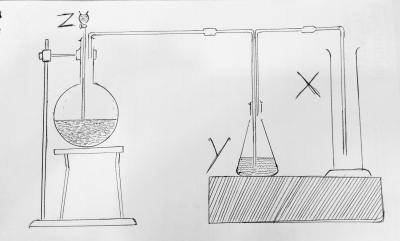
The diagram above. Y is
- A. fused CaO
- B. H2O
- C. NaOH
- D. Concentrated H2SO4
Cu2S(g) + O2(g) → 2Cu + SO2(g)
What is the change in the oxidation number of copper in the reaction?
- A. 0 to + 2
- B. 0 to + 1
- C. + 1 to 0
- D. + 2 to + 1
The alkyl group is represented by the general formula
- A. CnH2n
- B. CnH2n − 2
- C. CnH2n + 1
- D. CnH2n + 2
The enzyme used in the hydrolysis of starch to dextrin and maltose is?
- A. lipase
- B. amylase
- C. invertase
- D. zymase
The reaction of halogens with alkanes in the presence of sunlight is an example of
- A. oxidation reaction
- B. addition reaction
- C. hydrogenation reaction
- D. substitution reaction
Calculate the amount in moles of silver deposited when 9650C of electricty is passed through a solution of silver salt [= 96500 Cmol-1]
- A. 0.05
- B. 10.80
- C. 10.00
- D. 0.10
Tin is unaffected by air at ordinary temperature due to its?
- A. Low melting point
- B. Weak electropositive character
- C. High boiling point
- D. White lustrous appearance
The mass of silver deposited when a current of 10A is passed through a solution of silver salt for 4830s is – (Ag = 108 F = 96500(mol-1)
- A. 54.0g
- B. 27.0g
- C. 13.5g
- D. 108.0g
The shape of ammonia molecules is
- A. trigonal planar
- B. octahedral
- C. square planar
- D. tetrahedral
\(^{226}_{88}Ra\) → \(^x_{86}Rn\) + alpha particle
- A. 226
- B. 220
- C. 227
- D. 222
When ΔH is negative, a reaction is said to be
- A. endothermic
- B. exothermic
- C. reversible
- D. ionic
The ideal gas laws and equations are true for all gases at
- A. low pressures and lower temperatures
- B. low temperatures and high pressures
- C. high pressures and high temperatures
- D. low pressure and high temperatures
The salt formed from a weak acid and a strong base hydrolyzes in water to form
- A. A saturated solution
- B. an acidic solution
- C. a buffer solution
- D. an alkaline solution