Which of the following products of biotechnology can be used as a fuel in place of petrol?
- A. Butane
- B. Ethanol
- C. Ethene
- D. Propanol
A substance responsible for the sour taste of unripe orange is
- A. Alkene
- B. Alkanol
- C. Alkanoic acid
- D. Alkanoate
Which of the following process does not take place in domestic water treatment?
- A. Chlorination
- B. Flocculation
- C. Neutralization
- D. Sedimentation
Which of the following substances is a heavy chemical?
- A. Ammonia
- B. Barim Hydroxide
- C. Hydrochloric acid
- D. Tetraoxosulphate(VI)acid
Which of the following compounds is a secondary alkanol?
- A. Ethanol
- B. 2-methylbutan-2-ol
- C. 3-methylpentan-2-ol
- D. Propan-1-ol
Starch could be converted to glucose by the process of
- A. Condensation
- B. Dehydration
- C. Fermentation
- D. Hydrolysis
Which of the following reactions would take place when concentrated sodium hydroxide solution is added to palm oil?
- A. Esterification
- B. Neutralization
- C. Polymerization
- D. Saponification
A compound has an empirical formular CH2O and molecular mass of 90.
[H = 1.00, C = 12.0, O = 16.0]
- A. C4H10O2
- B. C3H10O2
- C. C3H6O3
- D. C2H2O4
The complete hydrogenation of benzene gives
- A. Cyclohexene
- B. Cyclohexane
- C. Hexene
- D. Hexane
Which of the following metals is the strongest reducing agent?
- A. Sodium
- B. Silver
- C. Potassium
- D. Copper
Consider the following reaction equation:
CuO(s) + H2(g) \(\to\) Cu(s) + H2O(1)
Which substance is oxidized?
- A. Cu
- B. CuO
- C. H2
- D. H2O
Which of the following statements about an electrochemical cell is correct? Oxidation occurs
- A. At the anode
- B. At the cathode
- C. Through the salt bridge
- D. In the aqueous solution
A change in the temperature of a saturated solution disturbs the equilibrium between the
- A. Undissolved solute and the solvent
- B. Dissolved solute and the solvent
- C. Dissolved solute and the undissolved solute
- D. Dissolved solute and the solution
A substance which dissolves readily in organic solvent would
- A. Be a covalent compound
- B. Have strong electrostatic forces of attraction
- C. have a high melting point
- D. Conduct electricity in molten state
Which of the following compounds is the least soluble in water?
- A. CaCl2
- B. CaSO4
- C. NaCl
- D. Na2SO4
Solubility is practically applied in
- A. Fractional distillation
- B. The determination of pH
- C. The determination of saturation in hydrocarbons
- D. Solvent extraction
Which of the following oxides can be reduced by hydrogen?
- A. Aluminium oxide
- B. Magnesium oxide
- C. Sodium oxide
- D. Silver oxide
An example of an acid salt is
- A. CH3COOONa
- B. Mg(OH)Cl
- C. NaHSO4
- D. (NH4)2SO4
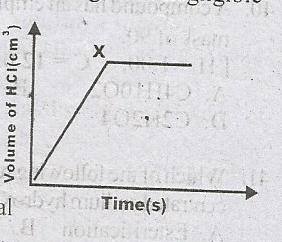
The following diagram illustrates the rate curve that was obtained when Mg reacted with excess dilute HCl.
The diagram became horizontal at X because
- A. The reaction was slowed down
- B. All the dilute HCl has reacted
- C. All the Mg has reacted
- D. Hydrogen gas is produced at a steady rate
Which of the following statements about intermolecular distances and cohesive forces between gas is correct? They are
- A. Both large
- B. Both negligible
- C. Constant and negligible
- D. Large and negligible
When a reaction is endothermic,
- A. Enthalpy change, \(\Delta\)H is negative
- B. Heat content of a product is less than the heat content of a reactant
- C. Heat content of reactants is less than the heat content of product
- D. The reaction is non-spontaneous


