The shape of the water molecule is_______?
- A. Linear
- B. Pyramidal
- C. Tetrahedral
- D. V-shaped
Which of the following arrangements shows increasing order of reactivity of the halogens?
- A. F2 > Cl2 > Br2 >I2
- B. I2 < Br2 < Cl2< F2
- C. F2 < Cl2 < Br2< I2
- D. I2 > Br2 > Cl2 > F2
An unsaturated solution differs from a saturated solution at a given temperature because it
- A. Cannot dissolve more solute
- B. Can hold more solute than it can actually dissolve
- C. Can still dissolve more solute at given temperature
- D. Form crystal more easily on cooling
Aluminium is used in the manufacture or aircraft because it
- A. Is hard and brittle
- B. Is light and resists corrosion
- C. Has high density and conducts electricity
- D. Is malleable and ductile
Metals can be stretched into wires because they are
- A. Ductile
- B. Good conductors
- C. Lustrous
- D. Malleable
The mass of potassium hydroxide required to make 300.ocm3 of 0.4 moldm-3 solution is {KOH = 56.0}
- A. 26.88g
- B. 13.44g
- C. 6.72g
- D. 3.36g
Chemicals that are produced in small quantities and with very degree of purity are
- A. Bulk chemicals
- B. Fine chemicals
- C. Heavy chemicals
- D. Light chemicals
The boiling points of HF, H2O and NH3 increase in the order of
- A. NH3
- B. H2O < HF
- C. HF < NH3 < H2O
- D. NH3 < HF < H2O
When element \(_{20}\)Y combines with element \(_8\)Z, it forms
- A. A covalent compound , YZ is formed
- B. A covalent compound, ZY is formed
- C. An ionic compound, YZ is formed
- D. An ionic compound, ZY is formed
How many electrons are present in \(^9_4Be^{2+}\)?
- A. 2
- B. 4
- C. 5
- D. 6
An atom X consist of 6 protons, 6 electrons and 7 neutrons. Which of the following representations of the atom is correct?
- A. 136X
- B. 137X
- C. 196X
- D. 197X
The following atoms of carbon 126C, 136C and 146C can be described as?
- A. Allotropes
- B. Isomers
- C. Isotopes
- D. Isotones
N2O4(aq) \(\rightleftharpoons\) 2NO2(g) \(\bigtriangleup\)H = +ve
In the reaction above, an increase in temperature will
- A. Increase the reactant production
- B. Increase the value of the equilibrium constant
- C. Shift the equilibrium to the left
- D. Decrease the value of the equilibrium constant
CH4(g) + CI2(g) \(\to\) CH2CI(s) + HCIg
The major factor that influences the rate of the reaction above is
- A. Concentration
- B. Catalyst
- C. Temperature
- D. Light
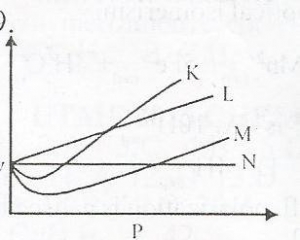
From the diagram above, an ideal gas can be represented by
- A. K
- B. M
- C. L
- D. N
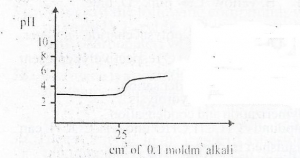
The curve depicts titration between a strong acid of pH
- A. Strong acid and strong base
- B. Strong acid and weak base
- C. Weak acid and weak base
- D. Weak acid and strong base
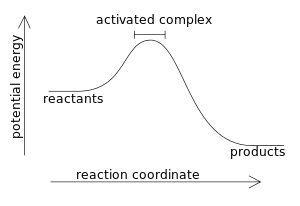
The diagram represents
- A. A spontaneous reaction
- B. An exothermic reaction
- C. A non-spontaneous
- D. An endothermic reaction
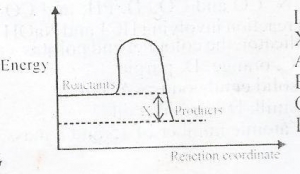
In the diagram above, X is the
- A. Enthalphy
- B. Activated complex
- C. Activation energy
- D. Enthalphy change
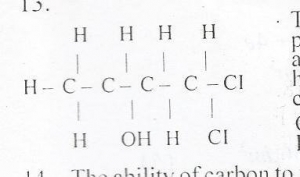
The functional groups present in the compound above are
- A. Alkene and halo-group
- B. Hydroxyl and chloro-group
- C. Alkene and chloro-group
- D. Hydroxyl and halo-group
n monosaccharide \( \underset{Q}{\stackrel{P}{\rightleftharpoons}} \) polysaccharide –n water.
In the process above, P and Q respectively represent
- A. Condensation and hydrolysis
- B. Fermentation and condensation
- C. Polymerization and hydrolysis
- D. Polymerization and condensation
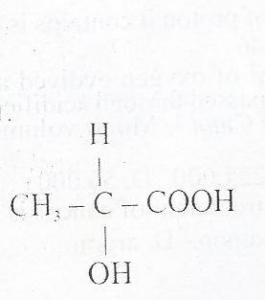
The compound above exhibits
- A. Geometric isomerism
- B. Positional isomerism
- C. Structural isomerism
- D. Optical isomerism


