Which of the following classes of organic compounds undergoes rapid oxidation with bromine water?
- A. alcohols
- B. alkenes
- C. carboxylic acids
- D. esters
- E. alkanes
Detergents are more advantageous than soaps in hard water districts because
- A. on treatment with hard water they either form soluble calcium salts or do not react with calcium ion
- B. on treatment with hard water they form soluble calcium salts
- C. on treatment with hard water they form sodium salts
- D. on treatment with hard water they are converted into soaps
- E. on treatment with hard water they form more lather than soaps does
Which of the following derivatives of nitrogen affords an aqueous solution having a pH of less that 7?
- A. N2O
- B. NO
- C. NO2
- D. NaNO3
- E. NH3
When copper (ii) sulphate solution is treated with excess ammonium hydroxide, a precipitate is first formed, and then this dissolves to give a deep blue solution.
This deep blue solution is a?
- A. Cu(OH)2
- B. CuSo4.5H2O
- C. (NH4)2SO4
- D. Cu(NH3)4(OH)2
- E. CuSO4.(NH4)2SO4.2H2O
Which of the following tests would confirm-unequivocally that a solution X contain the sulphate ion if it gives with?
- A. silver nitrates solution, a white precipates insoluble in dilute HNO3
- B. barium chloride solution, a white precipitate insoluble in dilute H2SO4
- C. barium chloride solution, a white precipitate soluble in dilute HCl
- D. barium chloride solution a white precipitate soluble in dilute ammonia
- E. barium chloride solution a white precipitate insoluble in dilute HCl
The following arrangement of elements in order of decreasing number of outer electrons; One of the elements is wrongly placed. Which is it? ALUMINIUM, CHLORINE, SILICON, MAGNESIUM AND SODIUM.
- A. chlorine
- B. aluminium
- C. silicon
- D. magnesium
- E. sodium
Which of the following represents a precipitation reaction?
- A. H2SO4 + NaCl \(\to\) NaHSO4 + HCl
- B. Fe +H2SO4 \(\to\) FeSO4 + H2
- C. 4HCl + MnO2 \(\to\) MnCl2 + 2H2O + Cl2
- D. Na2SO4 + pb(NO3) \(\to\) PbSO4 + 2NaNO
- E. CuO +2HCl \(\to\) Cl2 + H2O
1.00g of mixture of calcium carbonate and calcium oxide liberated 0.33g of carbon dioxide on strong heating. The percentage of calcium oxide in the mixture is? (Ca = 40, C = 12, O = 16)
- A. 5
- B. 15
- C. 25
- D. 35
- E. 50
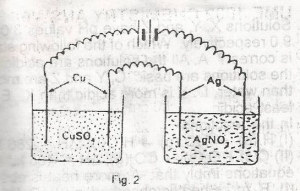
In the experiment above, a current was passed for 10 minutes, and 0.63g of copper was found to be deposited on the cathode of CuSO4 cells. The weight of silver deposited on the cathode of AgNO3 cell during the same period would be? [Cu = 63, Ag = 108]
- A. 0.54g
- B. 1.08g
- C. 1.62g
- D. 2.16g
- E. 3.24g
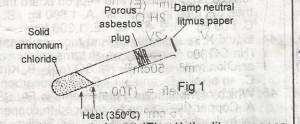
In the above experiment, the litmus paper will initially
- A. be bleached
- B. turn green
- C. turn red
- D. turn blue
- E. turn black
Copper sulphate solution is electolysed using platinum electrodes A. current of 0.193 amperes is passed for 2hrs. How many grams of copper are deposited? (cu = 63.5, F = 96500 coulombs)
- A. 0.457g
- B. 0.500g
- C. 0.882g
- D. 0.914g
- E. 1.00g
In what respect will two dry samples of nitrogen gas differ from each other if sample 1 is prepared by completely removing CO2 and O2 from air, and sample 2 is prepared by passing purified nitrogen(i)oxide over heated copper? sample 1 is
- A. purer than sample 2
- B. slightly denser than sample 2
- C. in all respects the same as sample 2
- D. colourless but sample 2 has a light brown colour
- E. slightly less reactive than sample 2
The boiling point of water, ethanol, toluene and butan-2-ol are 373.0k, 351.3k, 383.6k and 372.5k respectively. Which liquid has the highest vapour pressure at 323.0k?
- A. water
- B. toluene
- C. ethanol
- D. butan-2-ol
- E. none
Hydrogen is not liberated when trioxonitrate(v)acid reacts with zinc because
- A. zinx is not rendered passive by acid
- B. hydrogen produced is oxidized to water
- C. oxides of nitrogen are produced
- D. all nitrates are soluble in water
- E. trioxonitrate(v)acid is a strong acid
A certain volume of gas at 298k is heated such that its volume and pressure are now four times the original values. What is the new temperature?
- A. 18.6k
- B. 100.0k
- C. 298.0k
- D. 1192.0k
- E. 4768.0k
Some properties of chemical substances are mentioned below… i. sour taste ii. slippery touch iii. yields alkaline gas with ammonium salt iv. has pH less than 7 v. turns phenolphthalein pink.
Which of the above are NOT typical properties of alkalis?
- A. i, iv and v
- B. iv and v
- C. i and iv
- D. ii and v
- E. ii, iii and iv
3.0g of a mixture of potassium carbonate and potassium chloride were dissolved in a 25cm3 of this solution required required 40.00cm3 of 0.1M HCl for neutralization. What is the percentage by weight of K2CO3 in the mixture. (K = 39, O = 16, C = 12)
- A. 60
- B. 72
- C. 82
- D. 89
- E. 92
Given that the molecular mass of iron is 56 and that of oxygen is 16, how many moles of iron(iii)oxide will be contained in 1kg of the compound?
- A. 25.0 moles
- B. 12.5 moless
- C. 6.25 moles
- D. 3.125 moles
- E. 0.625 moles
Hydrogen diffuses through a porous plug
- A. at the same rate as oxygen
- B. at a slower rate than oxygen
- C. twice as fast as oxygen
- D. three times as fast as oxygen
- E. four times as fast as oxygen
In the experiment, which of the following observations would suggest that a solid sample is a mixture? The
- A. solid can be ground to a fine powder
- B. density of the solid is 2.25gdm-3
- C. solid begins to melt at 573k but is not completely melted until 648k
- D. solid absorbs moisture from the atmosphere and turns into liquid
- E. solid melts at 300k
0.499g 0f CuSO4.xH2O when heated to constant weight gave a residue of 0.346g. the value of x is? (Cu = 63.5, S = 32.0, O = 16, H = 1)
- A. 0.5
- B. 2.0
- C. 3.0
- D. 4.0
- E. 5.0


