ANWSER
Question 1(a):
The photochemical reaction of two molecules of ethylene (likely a typo; ethane lacks π bonds) proceeds via a one-step concerted mechanism. Under UV light, the π electrons in ethylene are excited, allowing a [2+2] cycloaddition. This reaction is suprafacial and concerted, forming cyclobutane without intermediates. Thermal [2+2] cycloadditions are symmetry-forbidden (4π electrons), but photochemical excitation overcomes this barrier, enabling a concerted pathway.
Question 1(b):
For molecules with 4n π electrons (e.g., [4+4] cycloaddition), a thermal concerted cycloaddition is forbidden. However, a stepwise diradical-mediated dimerization can occur. For example, two conjugated dienes (4π each) react via diradical intermediates, forming a bicyclic product. The reaction involves two steps: (1) bond formation at one site, generating a diradical, and (2) bond formation at the second site. Products include cyclooctane derivatives or fused bicyclic compounds.
Question 1(c):
The [3,3] Claisen sigmatropic rearrangement of an allyl vinyl ether produces a γ,δ-unsaturated carbonyl compound. For example, allyl phenyl ether rearranges to 2-allylphenol (or a carbonyl derivative if substituents are present). The mechanism involves a concerted shift of the allyl group and reorganization of π bonds.
—
Question 2(a):
Cyclohexane → Lactone:
1. Dehydrogenate cyclohexane to cyclohexene (using a catalyst).
2. Epoxidize cyclohexene (e.g., with mCPBA) to form cyclohexene oxide.
3. Acid-catalyzed ring-opening of the epoxide to yield trans-1,2-cyclohexanediol.
4. Oxidize one -OH group to -COOH (e.g., with KMnO₄/H⁺).
5. Lactonization under acidic conditions forms a δ-valerolactone (5-membered cyclic ester).
Equation:
Cyclohexane → Cyclohexene → Cyclohexene oxide → trans-1,2-Cyclohexanediol → γ-Hydroxycyclohexanecarboxylic acid → δ-Valerolactone.
Question 2(b):
(i) Spectroscopy:
– IR: Loss of -OH (3300 cm⁻¹) and appearance of ester C=O (1740 cm⁻¹).
– ¹H NMR: Absence of -OH proton; signals for ester carbonyl and cyclic structure.
(ii) Classical Analysis:
– Saponification: Hydrolyze lactone with base, titrate to determine ester content.
Question 2(ci):
Reaction: Dehydration of an alcohol (e.g., cyclohexanol) with H₂SO₄.
Products:
– Major: Cyclohexene (Zaitsev product).
– Minor: Less substituted alkene (if applicable).
Question 2(d):
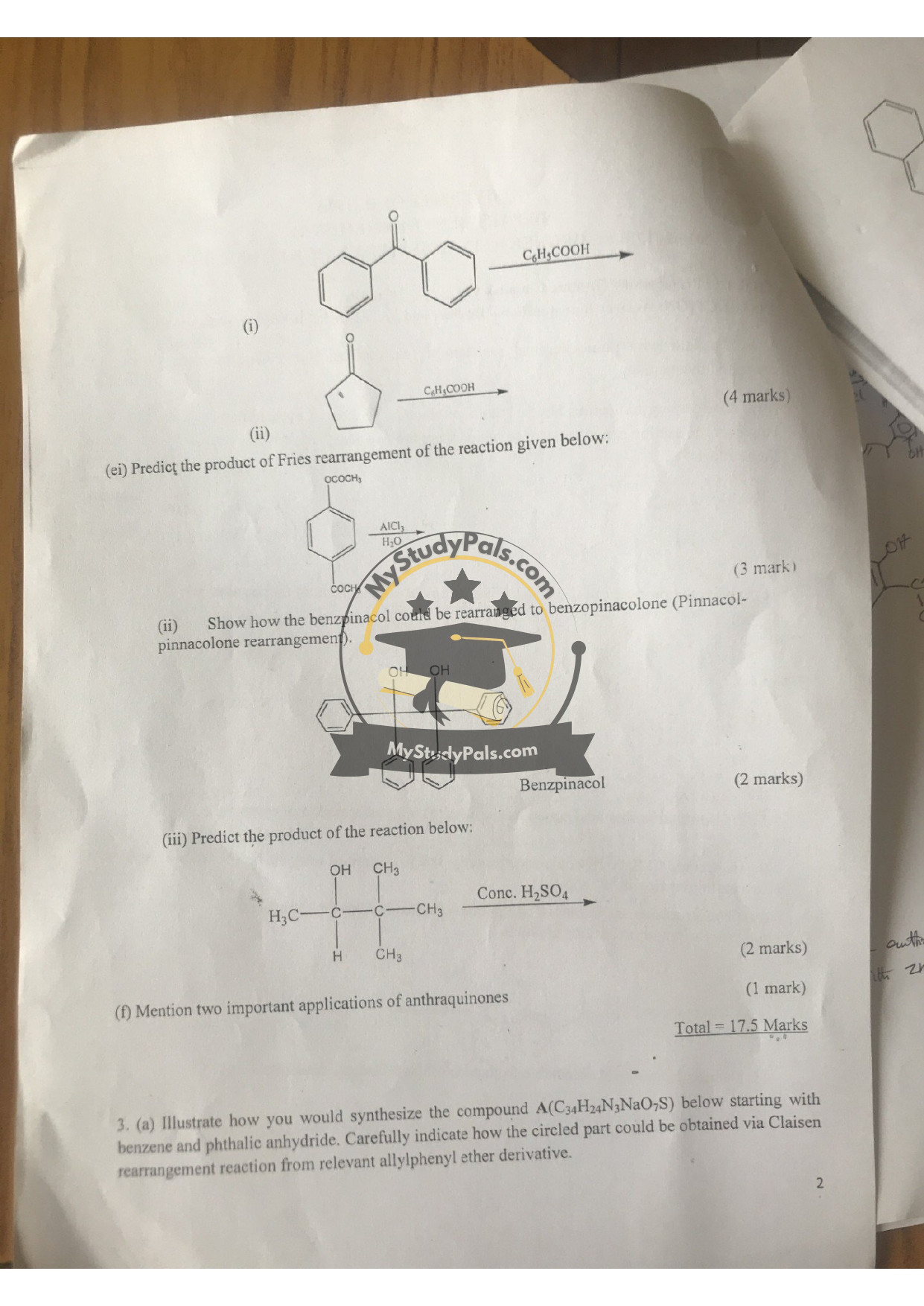
(i) C₆H₅COOH (Benzoic acid) → No reaction specified; likely esterification or decarboxylation.
(ii) Fries Rearrangement (AlCl₃/H₂O): Rearranges phenyl acetate to para-hydroxyacetophenone.
(iii) Benzpinacol → Benzopinacolone: Acid-catalyzed dehydration forms a ketone (migration of phenyl groups).
(iv) Dehydration with H₂SO₄: Converts alcohol (e.g., (CH₃)₂CHOH) to alkene ((CH₃)₂C=CH₂).
Question 2(f):
Applications of Anthraquinones:
1. Dyes (e.g., alizarin for textiles).
2. Laxatives (e.g., senna glycosides).
—
Question 3(a):
Synthesis of A (C₃₄H₂₄N₃NaO₇S):
1. Sulfonate benzene to form benzenesulfonic acid.
2. Condense with phthalic anhydride to create a sulfonated phthalein.
3. Introduce an allyl group to form an allyl phenyl ether.
4. Claisen Rearrangement: Heat to rearrange the allyl phenyl ether into a γ,δ-unsaturated carbonyl compound.
Question 3(b):
Replacing β-naphthol with 3-methyl α-naphthol introduces a methyl group at the α-position of the naphthalene ring, altering substitution patterns in the final product.
Question 3(c):
Stimulating Transformation to B: Use acid catalysis or thermal conditions to favor specific carbocation rearrangements or ring-expansion pathways.
Question 3(d):
Spectroscopic Confirmation:
– UV-Vis: Diazonium salts absorb at ~400 nm; azo products show intense visible absorption.
– IR: Loss of NH₂ peaks (3300 cm⁻¹); appearance of N=N (1600 cm⁻¹).
Question 3(e):
Ring Expansion: Acid-catalyzed dehydration of H₂C-CH₂-C-OCH₃ generates a carbocation. A hydride shift or methyl migration leads to cycloheptanone (7-membered ring).
—
Question 4(a):
Acetoacetic Ester Synthesis:
1. Enolate Formation: Treat ethyl acetoacetate with NaOEt.
2. Alkylation: React with 1-bromobutane.
3. Second Alkylation: Repeat with another equivalent.
4. Hydrolysis: Acidic hydrolysis yields β-keto acid.
5. Decarboxylation: Heat to form 3-pentanone (CH₃COCH₂CH₂CH₃).
Question 4(b):
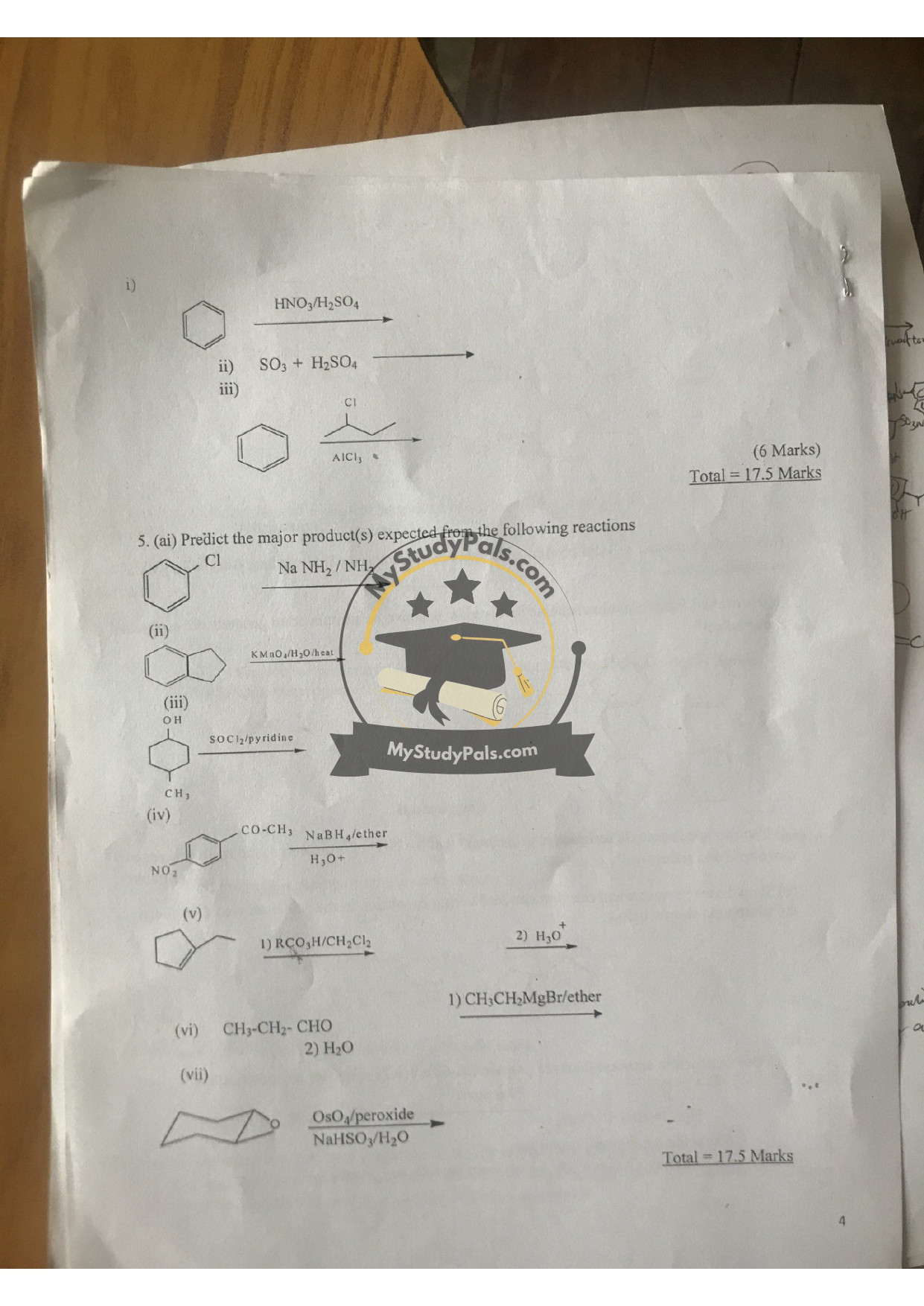
Major Products:
(i) Cl + NaNH₂/NH₃: Alkene (E2 elimination).
(ii) KMnO₄/H₂O/Heat: Carboxylic acid (oxidative cleavage).
(iii) OH + SOCl₂/pyridine: Alkyl chloride.
(iv) COCH₃ + NaBH₄: Secondary alcohol.
(v) RCO₃H → H₃O⁺: Diol (epoxide ring-opening).
(vi) CH₃CH₂CHO + H₂O: Aldol product (if basic) or hydrate.
(vii) OsO₄/NaHSO₃: Vicinal diol (syn addition).
—
Question 5(ai):
Major Products:
(i) Cl + NaNH₂/NH₃: 1-Alkyne (dehydrohalogenation).
(ii) KMnO₄/H₂O/Heat: Adipic acid (oxidation of cyclohexene).
(iii) OH + SOCl₂: Alkyl chloride.
(iv) COCH₃ + NaBH₄: CH₃CH(OH)CH₂CH₂CH₃.
(v) RCO₃H → H₃O⁺: Trans-diol.
(vi) CH₃CH₂CHO + H₂O: No reaction (hydration equilibrium).
(vii) OsO₄/NaHSO₃: cis-1,2-Diol.